Delving into the fundamentals of anaerobic digestion
On the heels of my previous post, I wanted to write something else related to the science of wastewater treatment. The two papers I discuss below are ones that I've read in the past, but haven't shared any notes on before.
The papers I discuss below are written by a group of researchers from Wageningen University—famous around the world for its agriculture expertise, and also possessing a very strong history of wastewater treatment research. (The Netherlands in general is strong in all sorts of water-related engineering, which is part of the reason it's high on my list of countries I'd like to visit). These two papers are both tightly-focused review papers and both are very well written. They deal with the concept that anaerobic digestion requires a group of microorganisms to play different roles, and, because there is only a small amount of chemical energy available, electrons actually end up getting transferred between species.
The following are my notes and excerpts from these two papers:
"Interspecies electron transfer in methanogenic propionate degrading consortia"
- Authors: F.A.M.de Bok, C.M.Plugge, and A.J.M.Stams
- Journal: Water Research (2004)
Abstract:
Propionate is a key intermediate in the conversion of complex organic matter under methanogenic conditions. Oxidation of this compound requires obligate syntrophic consortia of acetogenic proton- and bicarbonate reducing bacteria and methanogenic archaea. Although H2 acts as an electron-carrier in these consortia, evidence accumulates that formate plays an even more important role. To make energy yield from propionate oxidation energetically feasible for the bacteria and archaea involved, the concentrations of H2 and formate have to be extremely low. On the other hand, the diffusion distance of these carriers has to be small to allow high propionate conversion rates. Accordingly, the high conversion rates observed in methanogenic bioreactors are due to the fact that the propionate-oxidizing bacteria and their methanogenic partners form micro-colonies within the densely packed granules.
Notes:
- During anaerobic digestion, organic compounds are broken down into simpler compounds—propionate (C2H5COO-) being a key one. Propionate is converted to acetate and hydrogen (and formate) by "acetogenic" bacteria; these products are then converted to biogas (CH4 + CO2) by methanogens (see here for an infographic I made that shows these steps).
- It is notable that the acetogenic step involves oxidation (the average oxidation state of carbon is 0 in acetate (and H2 has an oxidation state of 0 too, of course) but -2/3 in propionate), which is energetically unfavourable under anaerobic conditions.
- Table 1 shows the equations for relevant reactions. For the conversion of propionate to acetate and hydrogen (or acetate and formate) ΔG° > 0, but both hydrogenotrophic and acetoclastic methanogenesis have ΔG° < 0. So coupling these reactions together allows for the digestion of propionate to methane to yield energy for the microorganisms involved.
- The net reaction provides enough free energy (around 60 kJ/mol) to generate 1 unit of ATP, the molecule used for energy within cells. However, since 3 species are involved (i.e. acetoclastic bacteria, hydrogenotrophic methanogens, and acetoclastic methanogens), "interspecies electron transfer" is needed to share the available energy between them.
- Keeping the concentration of hydrogen (or formate) low makes acetogenesis more energetically favourable.
- Electrons are carried between cells by hydrogen or formate. The authors do some calculations on the flux of these carriers and they conclude that hydrogen is a more effective carrier over a short distance (it diffuses faster) but formate is a more effective carrier over a longer distance (it has better solubility in water). This is probably relevant for the differences between anaerobic bacteria in granules (where H2 would be the better electron carrier) versus flocs (where formate would be better).
- The achievable flux of an electron carrier affects the overall conversion rate of propionate to methane.
"Exocellular electron transfer in anaerobic microbial communities"
- Authors: Alfons J. M. Stams, Frank A. M. De Bok, Caroline M. Plugge, Miriam H. A. Van Eekert, Jan Dolfing, and Gosse Schraa
- Journal: Environmental Microbiology (2006)
Abstract:
Exocellular electron transfer plays an important role in anaerobic microbial communities that degrade organic matter. Interspecies hydrogen transfer between microorganisms is the driving force for complete biodegradation in methanogenic environments. Many organic compounds are degraded by obligatory syntrophic consortia of proton-reducing acetogenic bacteria and hydrogen-consuming methanogenic archaea. Anaerobic microorganisms that use insoluble electron acceptors for growth, such as iron- and manganese-oxide as well as inert graphite electrodes in microbial fuel cells, also transfer electrons exocellularly. Soluble compounds, like humic substances, quinones, phenazines and riboflavin, can function as exocellular electron mediators enhancing this type of anaerobic respiration. However, direct electron transfer by cell–cell contact is important as well. This review addresses the mechanisms of exocellular electron transfer in anaerobic microbial communities. There are fundamental differences but also similarities between electron transfer to another microorganism or to an insoluble electron acceptor. The physical separation of the electron donor and electron acceptor metabolism allows energy conservation in compounds as methane and hydrogen or as electricity. Furthermore, this separation is essential in the donation or acceptance of electrons in some environmental technological processes, e.g. soil remediation, wastewater purification and corrosion.
Notes:
- This paper is based on the same fundamental principle as the previous one (i.e. transfer of electrons between cells in anaerobic environments) but takes it in some different directions.
- The authors refer to part of the anaerobic community as an "obligatory syntrophic consort[ium]" that includes acetogens that reduce protons (i.e. H+ → H2) and methanogens that consume H2.
- Figure 1, a portion of which I've clipped and pasted below, provides a useful visual summary of the paper. (A) is how most microorganisms work where electrons are transferred within a cell from a substrate (electron donor) to an electron acceptor (e.g. oxygen under aerobic conditions). (B) represents a typical situation in anaerobic digestion, where electrons are transferred (with hydrogen as a carrier, although it could also be formate, as discussed above and in the current paper) between different species.
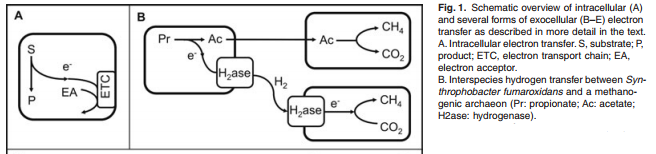
- Dechlorinating bacteria and sulfate-reducing bacteria can compete with methanogens for hydrogen under some circumstances.
- Aside from the production of methane, inorganic chemical species like Fe(III) and Mn(IV) can be reduced in anaerobic environments. Electrons can be transferred from bacteria to these electron acceptors by "redox shuttles" such as quinone functional groups or directly (in some cases) by conductive appendages.
- Finally, electrons can also be transferred to electrodes rather than to another microorganism or an oxidized chemical species. This is the principle behind microbial fuel cells.